Acrylic material test report
Item specifics
- Expiration Date
- 2023/10/15 - 2024/10/15
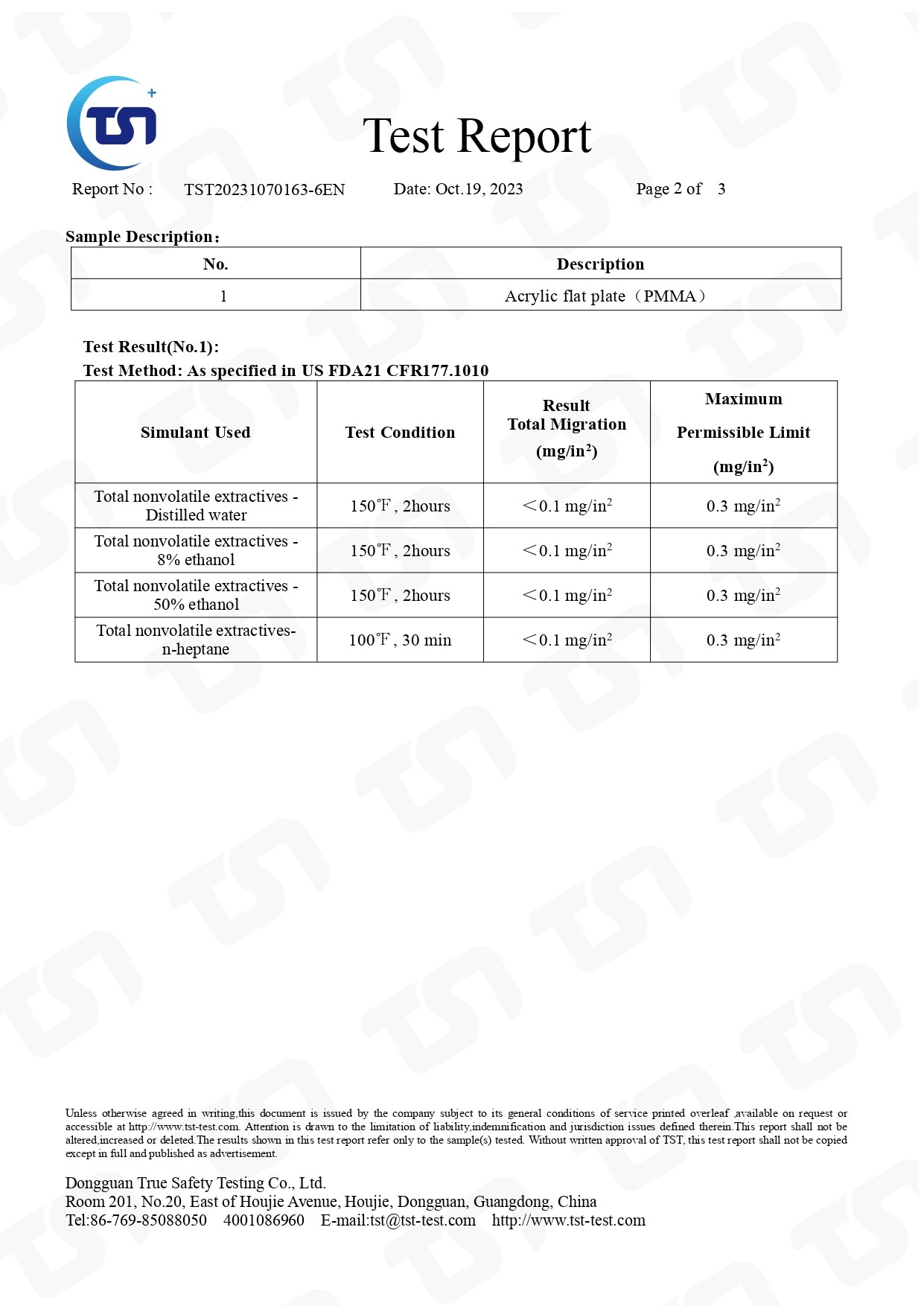
- No.
- TST20231070163-6EN
- Certification bodies
- TRUE SAFETY TESTING
- Organization Phone
- (86) 400-1086-960
Certificate description
FDA-US FDA21 CFR177.1010-PASS
US FDA 21 CFR 177.1010 is a regulation that addresses the use of "indirect food additives: polymers" by the United States Food and Drug Administration (FDA). This regulation provides specifications and guidelines for the use of certain polymers in contact with food.
Specifically, it outlines requirements for the safe use of polymers in various food-contact applications, such as packaging materials, containers, and other items that may come into contact with food during processing, storage, or transportation.
The regulation sets forth criteria for the types of polymers that may be used, as well as any additives or substances that may be present in the polymers. It also specifies limitations on the migration of substances from the polymers into food to ensure the safety of the food supply.
Manufacturers must comply with the requirements outlined in 21 CFR 177.1010 to ensure that their polymers meet FDA standards for food safety and do not pose any risk to human health. Compliance may involve testing, documentation, and adherence to specific manufacturing processes.