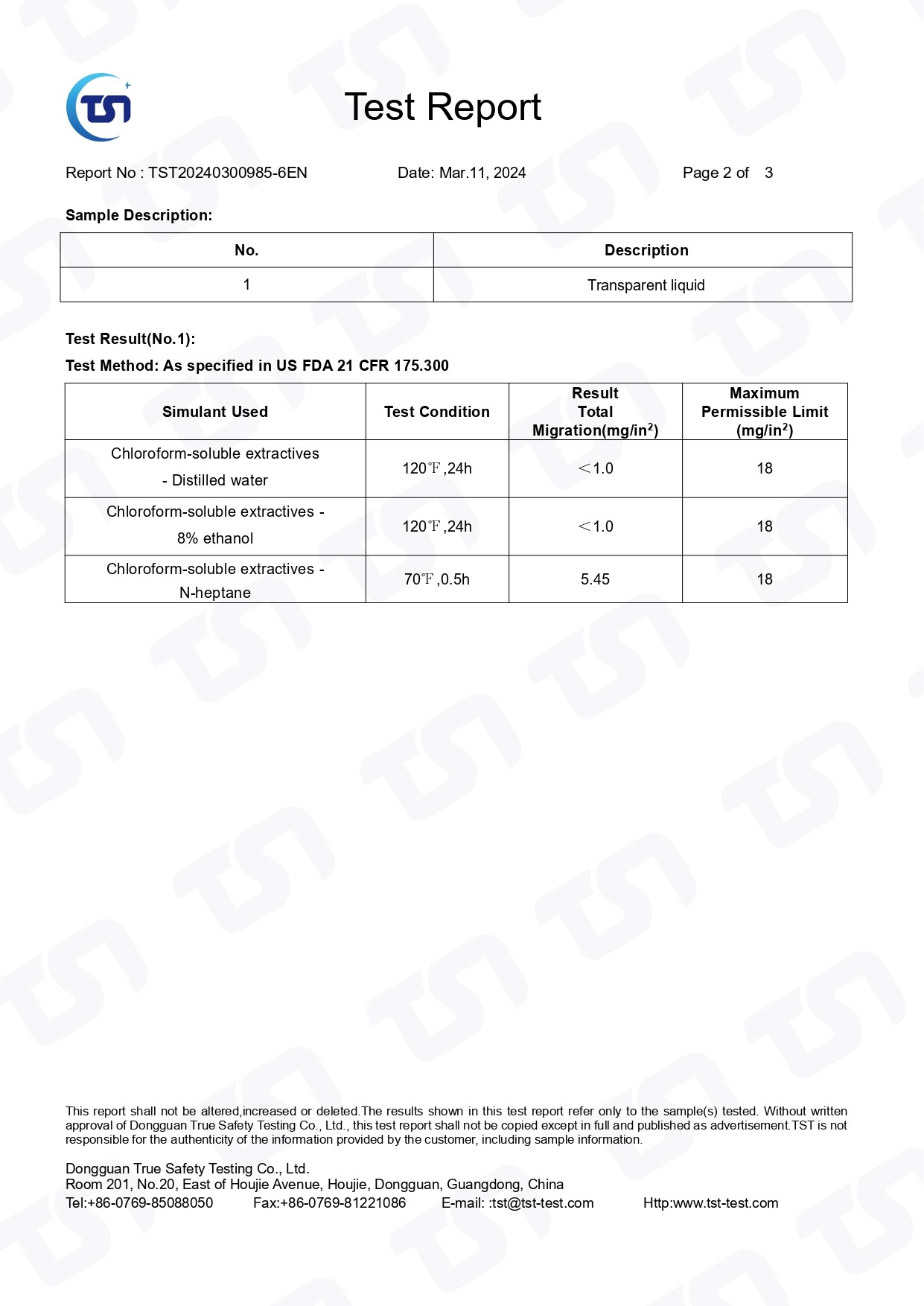
Acrylic glue test report
Item specifics
- Expiration Date
- 2024/3/7 - 2025/3/7
- No.
- TST20240300985-6EN
- Certification bodies
- TRUE SAFETY TESTING
- Organization Phone
- (86) 400-1086-960
Certificate description
FDA-US FDA 21 CFR 175.300-PASS
FDA 21 CFR 175.300 refers to a regulation set by the United States Food and Drug Administration (FDA). Specifically, it pertains to the materials and components used in the manufacturing of paper and paperboard products intended for direct food contact.
This regulation outlines the criteria and specifications for such materials and components, including permissible additives, substances, and limitations on their use to ensure the safety of food contact materials. Compliance with these regulations is necessary for manufacturers to ensure their products meet the FDA's standards for food safety.